Pathology and Digital Pathology Services

In an introductory consult, we will address your needs, such as model selection for your program's indication, experimental design for optimal pathology outcomes, and endpoint or advanced staining recommendations.
Our pathologists will then assist at every stage of the process for model development/proof-of-concept, efficacy, or safety studies.
Your program pathologist is accessible to answer clarifying questions, help integrate in vivo or ex vivo data, and prepare reports for regulatory submissions
Are we on the right track with this model? Does it exhibit the features we're hoping for? Is it similar to those we see in the clinic? What are the driving cell types and how should we continue to characterisze them? What are appropriate IHC stains to pursue? Can we benefit from a quantified dataset?
In all cases, a pathologist is present to guide the execution and interpretation of the dataset. We are in the business of providing answers and believe contextualization of data is critical.
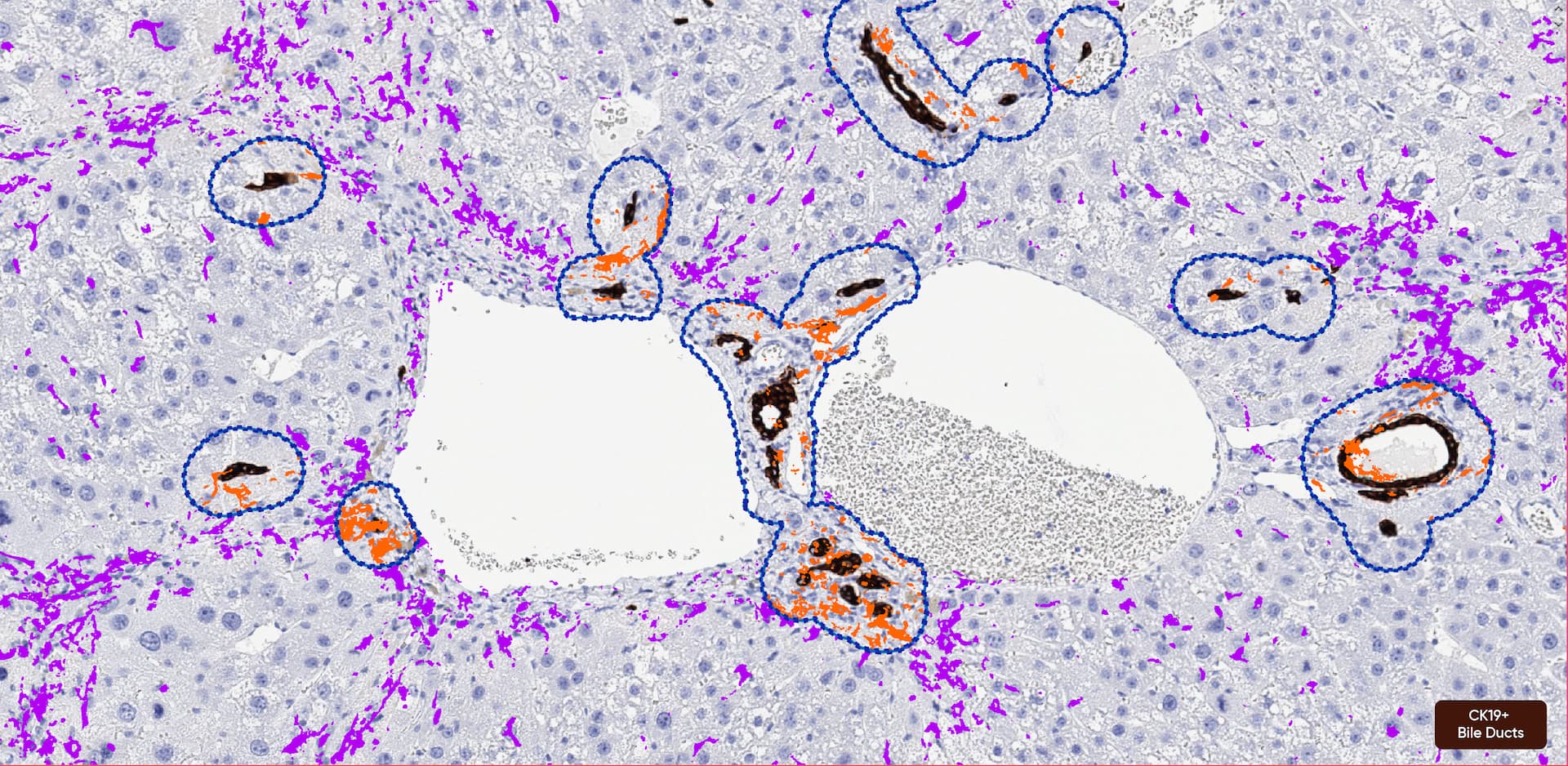
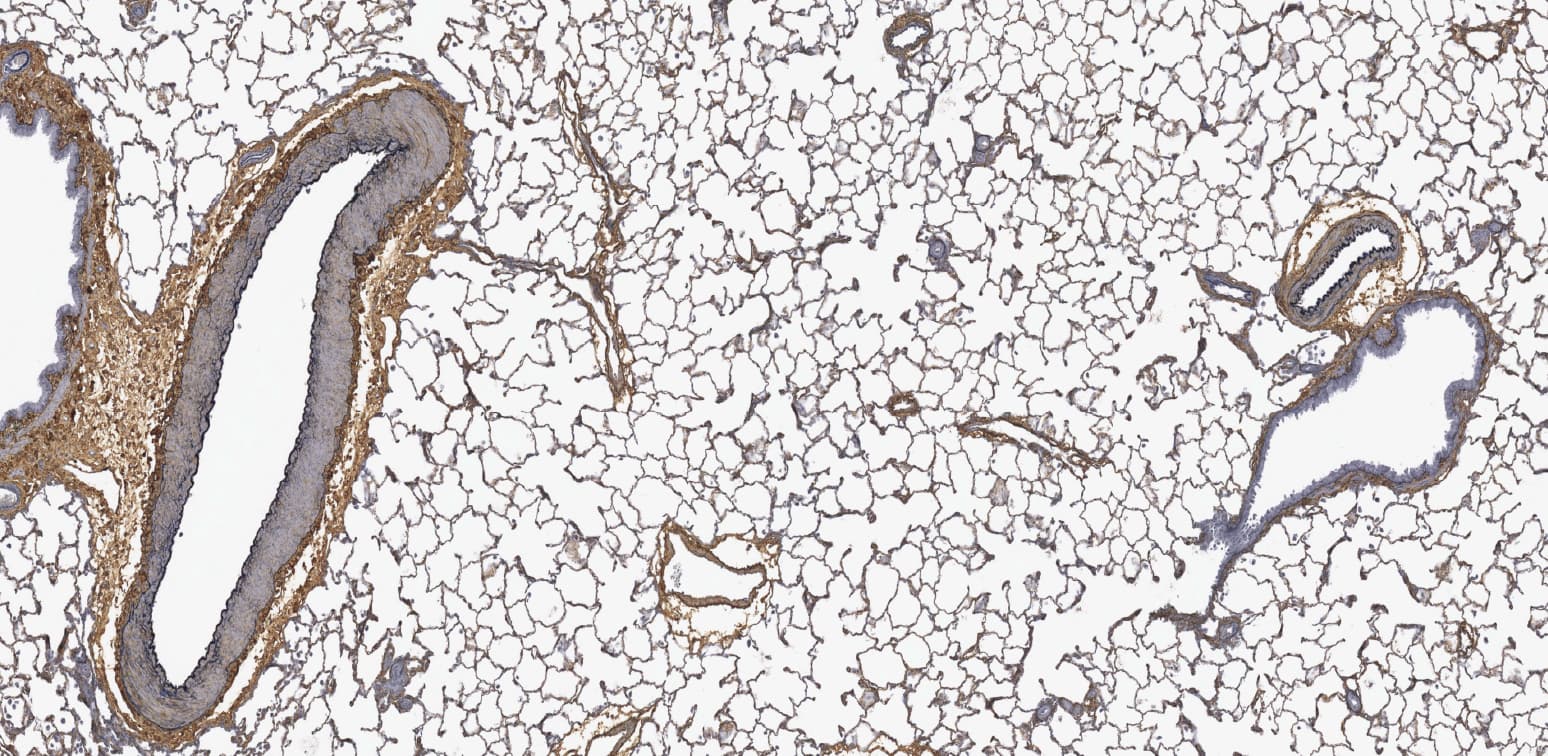
Image Analysis Capabilities
We start with a pathologist's review of an H&E and recommend antibodies to stain by IHC to highlight cell types of interest for quantification.
Quantitative solutions provide a more sensitive readout than manual scoring.
Using the same H&E or Special Stain for manual scoring, we can also quantify:
- Lipid content and steatosis
- Fibrotic endpoints- Inflammatory/necrotic foci
- Myocyte/Adipocyte Morphometrics
Endpoints include:
- General abundance screening
- H-scoring
- CISH/FISH


Reporting

Additional Services

Histology
.avif)
Advanced Staining

Consultations
Imaging

FAQs
The study protocol is the single best piece of information to share with the pathologist. If not available, a sample list and the experimental design, including model induction procedures, the number of groups, treatment information, and dosing paradigm are adequate.
Our pathologists and digital pathology team can discuss options for various endpoints to best suit your needs; however, some features are routinely analyzed by image analysis, such as quantifying cell/structure numbers, area of tissue affected, or intensity of a histochemical or immunohistochemical stain. Regardless of the type of evaluation, a pathologist will review the slides, interpret the data, and provide contextual information in the report.
We use Visiopharm to perform all of our quantification.
At minimum, an efficacy study should include 1) a model control (no disease induction) of the same age, strain, and sex as animals used in the study, and 2) a vehicle control with disease induction. These two controls allow the pathologist to determine the extent of disease induction compared to any background (non-model-associated) findings.Safety studies should always include a vehicle control of matched age, strain, sex, and administration route to allow for determination of test article-related findings.Our pathologists are happy to review study protocols and can answer questions regarding experimental designs.
We regularly accept and evaluate human tissue samples of nonclinical origin in addition to tissue from laboratory animals. Our veterinary pathologists can help answer non-diagnostic questions (e.g. cell type identification or immuno histochemistry interpretation) in human tissue. An MD pathologist is available to assist with questions that require diagnostic interpretation.
We are flexible and can provide a deliverable best-suited to your needs and timeline. We provide brief pathology reviews and datasets only on a limited basis, but all analyses and their quantified data are overseen by a pathologist regardless of formal reporting or not.
Yes, we have a selection of proofs of concept materials we have generated to help you visualize the endpoint and how that is interpreted by a pathologist. Please inquire if you have any specific needs outside of our sample data – we offer pilots to help foster understanding of our process and can work with you to identify what will be most informative for your study goals.
For most studies, blinded evaluation is not requested or performed.Masking of group/sample identities can be useful for preventing bias in studies with a clear hypothesis. For example, “Treatment A reduces the severity of inflammation in the DSS model of colitis.” If blinding is desired in such a case, we typically mask group identities, which are revealed following the evaluation, for appropriate reporting. For non-hypothesis-driven studies, such as toxicologic pathology/safety studies or model development, bias is desired. The pathologist’s role is to identify all possible findings and masking of group identities significantly limits the pathologist’s ability to do so. As such, blinding in this context is strongly discouraged.
An ACVP board-certified veterinary pathologist will evaluate nonclinical studies. If desired, an MD pathologist is available to evaluate human tissues. For efficacy studies, the pathologist will help choose the best scoring scheme for each model. Microscopic findings will be scored according to severity, following best practice guidelines. A published scoring scheme may also be utilized or modified to fit specific features of interest. For toxicologic pathology/safety studies, findings will be diagnosed according to standardized nomenclature and scored according to best practice guidelines from the Society of Toxicologic Pathology.
Yes! We perform a quality control check of all submitted materials and will inform you if additional materials, such as the tissue blocks, are required to meet your evaluation goals.












.webp)



.webp)