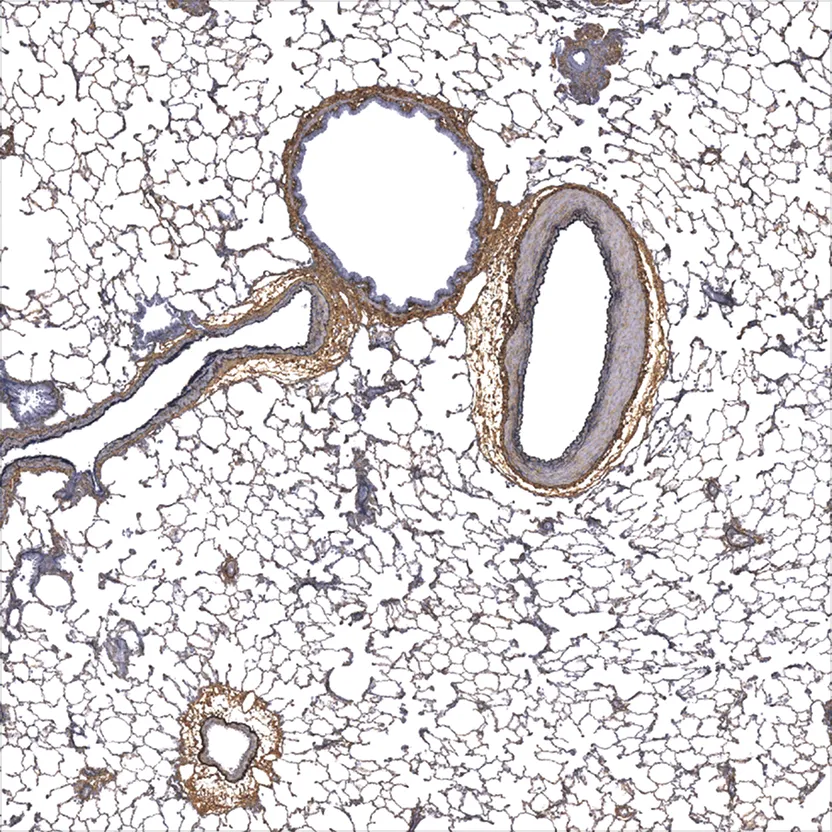
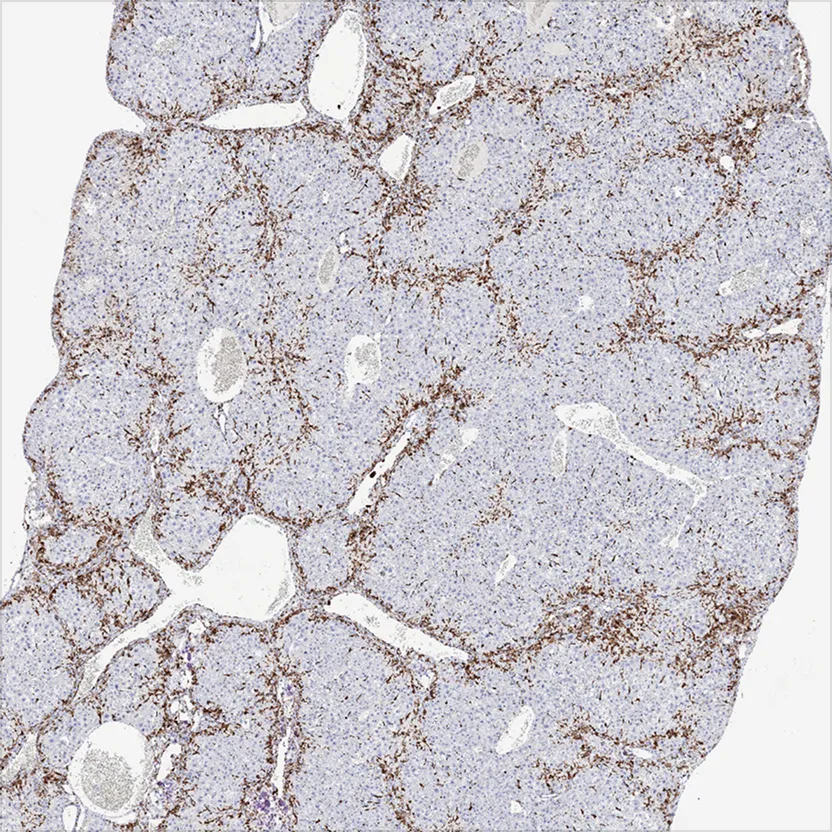
Advanced Staining

Method Development
Assistance with selection and procurement of commercially-available antibodies

Our scientists start with a series of standard protocols for staining on theLeica Bond platform. Selections of the most optimal protocols are overseen by a board-certified veterinary pathologist ensuring appropriate immunolabeling is present

Compilation of findings into a report with images for your records
.avif)
Routine Advanced Staining

Gallery
Imaging
- Leica (Aperio) AT2
- Olympus VS200
- Hamamatsu Nanozoomer
- 3D Histech Panoramic P250 Flash III
.avif)
Additional Services

Digital Pathology

Pathology
Consultations

Imaging

Histology
FAQs
There are pros and cons to both chromogenic and fluorescent immunostaining methods. Chromogenic IHC can provide a scientist or pathologist more morphological context that can enhance interpretation. Moreover, chromogenic immunostaining is not light sensitive, and labeling will tend to not degrade over time or with exposure to light. Fluorescent immunostaining can be better in instances when it is necessary to co-stain for two or more targets, and a high level of co-localization is expected. The appropriate staining method will depend on the goals of the specific project.
Many factors can affect ISH staining. Delayed fixation can cause RNA to degrade, and under-fixation can cause over-digestion of the tissue and result in poor morphology. Conversely, over-fixation can result in under-digestion of the tissue and result in no staining. Sample age can also affect RNA degradation, and slides should be cut fresh (<1 month) before ISH staining.
Generally, FFPE tissue is preferred, as the tissue morphology is better preserved and the sample is more shelf stable and not as susceptible to fluctuations in temperature. However, frozen tissue can be better in some circumstances. For example, formalin fixation can sometimes mask antigen epitopes; therefore, some antibodies may not be compatible with paraffin processing even with antigen retrieval. In these cases, frozen tissue may be a suitable alternative.
ISH and IHC staining methods can generally be combined easily as long as no protease steps are required in the ISH protocol.
Tissue should be fixed in formalin for no longer than 48 hours to prevent over-fixation. Most tissues can then be transferred to 70% EtOH or PBS + 0.01% Azide for long term storage. However, CNS (central nervous system) tissue should never be transferred to EtOH as this tissue is more sensitive to dehydration and should be transferred to PBS + 0.01% Azide.
Optimal IHC conditions will vary with each antibody, and an assay development phase is required for all new targets. A good starting concentration is 10ug/ml, which can then be adjusted up or down based on the labeling intensity and degree of non-specific background staining. Testing multiple antigen retrieval conditions, including enzymatic and heat-based retrievals, is recommended in all assay development phases.
Slides stained with chromogenic IHC or ISH methods can be stored at room temperature in a dry place, preferably in a slide box. Slides stained with fluorescent IHC or ISH methods should be stored in a refrigerator (4°C) in an opaque slide box to prevent exposure to light.
Choosing fluorophores will depend on the filter sets being used to visualize staining. Some filter sets overlap spectrally with others and require spectral unmixing software to distinguish. The following filter sets are the ones that we have found to be the most compatible with the least amount of spectral overlap: Dapi, FITC, TRITC (Cy3), Cy5, and Cy7. The FITC channel will generally demonstrate the most autofluorescence in FFPE tissue and should be reserved for the highest expressing target in the panel.
Preventing autofluorescence in FFPE tissue can be very difficult. Many commercially available autofluorescence quenchers are available, but the performance of such quenchers can vary. Generally, the best way to prevent autofluorescence is to use high quality antibodies with relatively intense expression so that exposure time when imaging can be minimized. Additionally, perfusing animals prior to tissue collection can reduce the number of erythrocytes, which are notorious for producing autofluorescence in most channels.